ATHN Projects and Research Studies—an Overview
ATHN is proud to partner with hemophilia treatment centers (HTCs)—our ATHN Affiliates—across the country on a number of innovative projects. We collaborate with researchers to facilitate a variety of studies through our federally funded HTCs, each study drawing from ATHN’s secure data resources to gain a better understanding of the issues affecting people with bleeding and clotting disorders. We also offer data and statistical services, as well as study implementation and support services, scientific leadership, and funding opportunities.
Introducing ATHN Transcends, a cohort-based study to streamline data collection and administration
The impact of ATHN’s success in leading research studies has resulted in over 15 separate studies. While we are proud of this accomplishment, it has also increased administrative and financial challenges for our ATHN Affiliates. To address the need for greater efficiency and ease, we’ve developed ATHN Transcends, a new natural history cohort study that will serve as the base study for streamlining and harmonizing data collection, as well as reducing the number of contracts and IRB submissions across multiple ATHN research studies. In addition, biospecimens will be collected in ATHN Transcends to be stored in the ATHN Research Biorepository for future research.
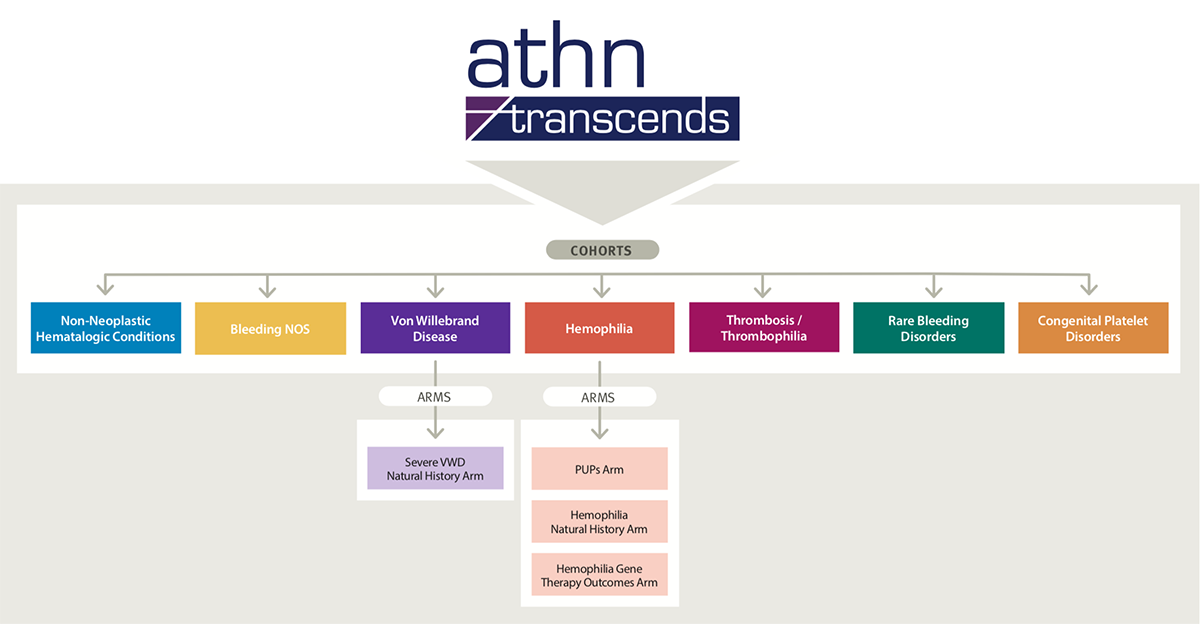
As the diagram below shows, ATHN Transcends is organized into 7 cohorts: Hemophilia, Von Willebrand Disease, Thrombosis/Thrombophilia, Non-Neoplastic Hematologic Conditions, Bleeding Not Otherwise Specified (NOS), Rare Bleeding Disorders, and Congenital Platelet Disorders. Individual “arms” fit within the cohorts. This umbrella design is modeled after successful National Cancer Institute (NCI)-supported groups and industry protocols.
To learn more about ATHN Transcends, visit ClinicalTrials.gov.
ATHN Projects and Research Studies
ATHN Transcends: A Natural History Cohort Study of the Safety, Effectiveness, and Practice of Treatment in People with Non-Neoplastic Hematologic Disorders is a cohort study to determine the safety, effectiveness, and practice of therapies used in the treatment of participants with congenital or acquired non-neoplastic blood disorders and connective tissue disorders with bleeding tendency. Safety is measured according to European Haemophilia Safety Surveillance (EUHASS) standards. Participants are followed longitudinally for at least 15 years from the time of enrollment. Study arms are assigned to one of the following 7 cohorts:
Hemophilia
- Hemophilia Natural History Arm
- PUPs Arm
- Hemophilia Gene Therapy Outcomes Arm
Von Willebrand Disease
Thrombosis/Thrombophilia
- There are no ATHN Transcends study arms at this time.
Non-Neoplastic Hematologic Conditions
- There are no ATHN Transcends study arms at this time.
Bleeding Not Otherwise Specified (NOS)
- There are no ATHN Transcends study arms at this time.
Rare Bleeding Disorders
- There are no ATHN Transcends study arms at this time.
Congenital Platelet Disorders
- There are no ATHN Transcends study arms at this time.
The following research studies are not part of ATHN Transcends.
Closed studies:
- ATHN 1: CVD in Hemophilia Study
- ATHN 2: Factor Switching Study
- ATHN 3: Radionuclide Synovectomy
- ATHN 5 & 6: HCV Outcomes and Eradication Studies
Open studies:
The following research studies are not part of ATHN Transcends.
Open studies:
- ATHN 10: Rare Coagulation Disorders Project
- ATHN 12: ATHNdataset HAD Pilot Project (opening 2021)
The following research studies are not part of ATHN Transcends.
Closed studies:
Open studies:
- ATHN 15: DOAC Use in Pediatric Thrombosis Patients
These important projects are happening at HTCs all over the United States. If you are a patient interested in learning more about any of our current projects, talk to your HTC team.
If you are a provider and interested in working with ATHN on one of our current projects or a researcher interested in proposing a project, please email us at support@athn.org.
